

The U.S. Food and Drug Administration has approved a Pfizer/BioNTech coronavirus vaccine for children ages 5-11. Teenagers were allowed to be vaccinated back in May. Russia's version of Sputnik for teens is promised to be registered in November. Ilya Yasny, head of scientific expert reviews at Inbio Ventures, looks into the need to vaccinate children (after all, they tend to suffer from mild bouts of the coronavirus infection), and explains the difference between the American and Russian approaches to testing.
Is there a problem?
The first objection to vaccinating children is that they tend to suffer from mild cases of covid, so no matter how safe the vaccine is, its benefits do not outweigh its potential harm. It is also thought that it is better to get sick - so that a more reliable immunity develops. Seems logical?
Let's do a mental experiment - what would you say about such a medicine: 2 million people received it, 8300 of them were hospitalized, of whom 2,700 ended up in intensive care and almost 100 died. I think such a drug would have been immediately pulled from the market, and everyone involved would have been severely punished. I described the statistics on covid in children ages 5-11 in the US since the pandemic began. As we can see, getting sick is not the best way to protect yourself. By the way, in the US, covid ranks eighth as a cause of child mortality, or sixth if you count only the period when the delta variant became widespread, ahead of influenza and other respiratory diseases.
30% of hospitalized children had no comorbidities.
7-8% of children who got sick with covid, even in a mild form, have the same lingering post-covid symptoms as adults - fatigue, headache, insomnia, difficulty concentrating, muscle and joint pain, cough. In addition to direct health damage, children (as well as adults) spread the disease in the family, kindergartens and schools.
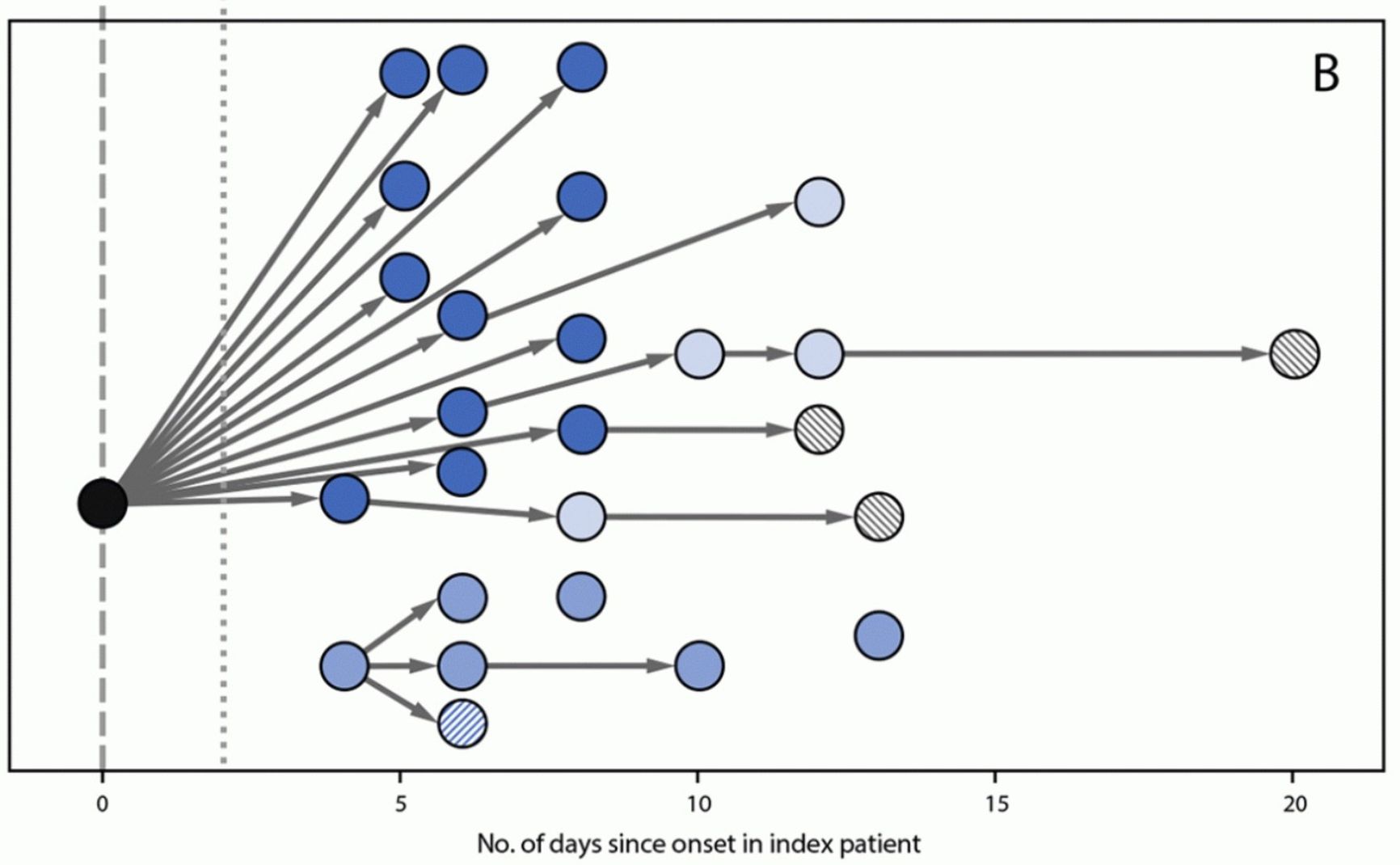
Figure 1. Covid distribution from patient zero to students and their parents in school
Centers For Disease Control And Prevention
Because of disease outbreaks, schools need to close, which of course is detrimental to education.
How effective is the vaccine?
In phase 2/3 clinical trials, the vaccine was administered to 1,500 participants aged 5-11 twice at three-week intervals, while 750 received a placebo. Within 120 days of vaccination three people became ill in the vaccine group, while 16 people became ill in the placebo group, i.e. the effectiveness of the vaccine was 90.7% [95% CI 67.7, 98.3].
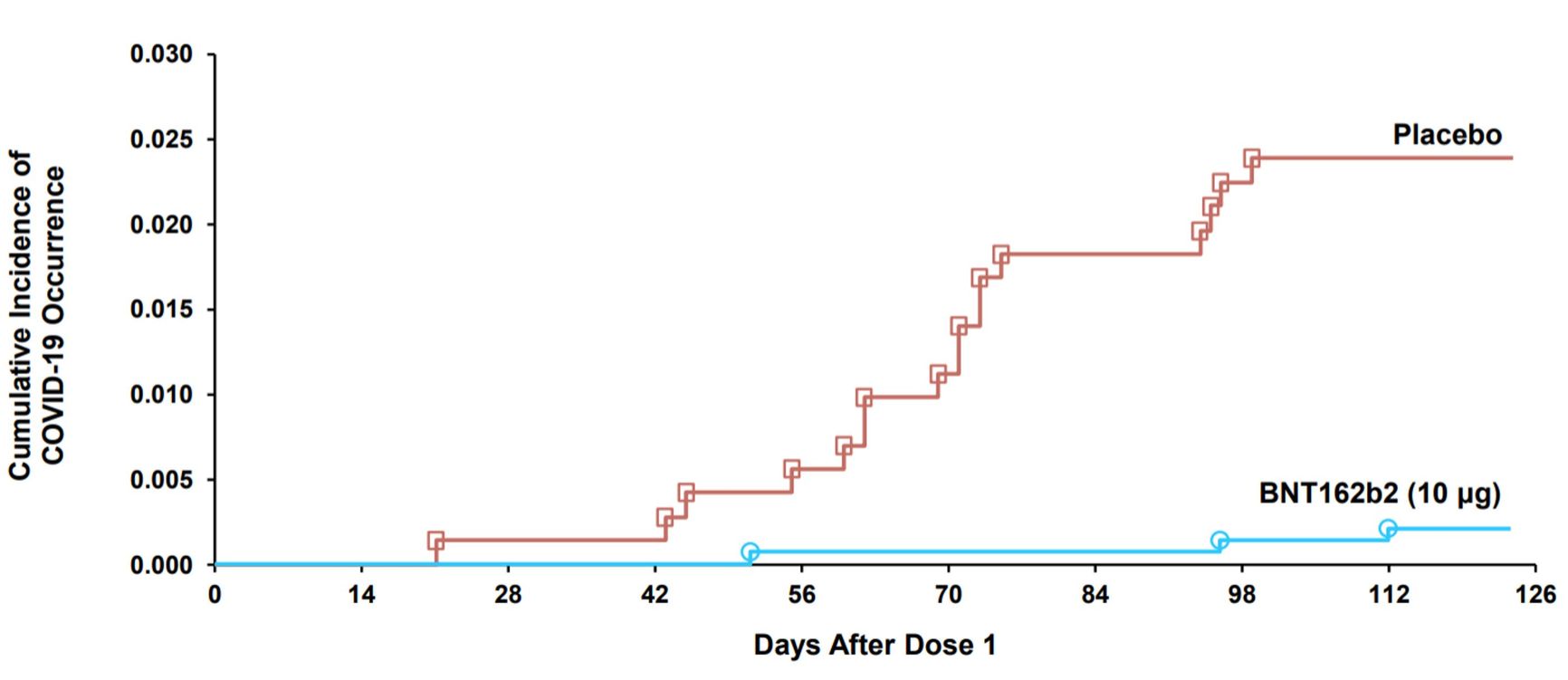
Figure 2. COVID-19 events accumulation curve after dose one in placebo and vaccine groups
Centers For Disease Control And Prevention
No severe cases, hospitalizations or deaths were recorded given such a small number of participants.
Thus, as for adolescents and adults, the vaccine has shown very high efficacy for children. A good result, considering that the dose of the active ingredient in the vaccine - 10 mcg - is three times smaller than in the «adult» version (and 10 times smaller than in the Moderna vaccine).
What about safety?
By the time of the filing with the US Food and Drug Administration (FDA), 3,082 children had received the vaccine. In general, the vaccine was more easily tolerated in children than in adolescents and adults. The incidence of injection site pain, fatigue and headache, and lymphadenopathy (swollen lymph nodes) was greater compared to placebo. All these adverse events were mild and subsided in 1-2 days.
The most important question on everyone's mind is the frequency of rare and delayed serious adverse events. In the older groups of patients vaccinated with Pfizer/BioNTech such an event was myocarditis - inflammation of the heart muscle. Before covid, myocarditis was generally more common in the 15-18-year-olds than in any other age groups, about 1.8 per 100,000, and resulted in deaths in 4-7% of cases. In the case of covid in children, the incidence of myocarditis is 0.02% to 0.08% of cases.
Since the start of Pfizer/BioNTech vaccination, there has been an increase in myocarditis, especially in men under 30 years of age. With 86 million doses administered, approximately 877 such cases (0.001%) were detected. Of those, 829 patients were hospitalized, 789 have already been discharged and most have recovered. Fortunately, no one died. Comparison of the normal and «covid» variants of myocarditis showed faster cardiac muscle recovery after vaccine-induced myocarditis.
The risk of vaccine-induced myocarditis is greater in boys aged 12-15: 60 cases of myocarditis have been reported per million doses administered. The risk increases after the second dose, so many countries have decided to limit vaccination to a single dose to strike a balance between safety and protection. In the US, 12 million 12-17-year-olds have already received the Pfizer/BioNTech vaccine, but a decision on a similar vaccine, Moderna, is still pending.
The incidence of common myocarditis is lower in children aged 5-11 than in adolescents, so there is an assumption that vaccine-induced myocarditis will also occur less frequently, especially given the reduced dose of the drug.
Pros and cons
As always, having considered the benefit-risk ratio based on the available information, independent experts in the United States have concluded that vaccination of children aged 5-11 can be recommended. Mathematical modelling showed that, depending on the scenario, vaccinating one million children could prevent one to three deaths, more than 60 ICU admissions, approximately 200 hospital admissions and 40,000-50,000 cases of disease given the scale of the current pandemic.
For those who say it was not worth it spending so much money on vaccine research and on «feeding» the greedy big pharma, the US epidemiological agency CDC has prepared a slide with previous childhood vaccine registrations: it turns out that the COVID-19 mortality rate has exceeded that of a number of other diseases that undoubtedly required vaccination.
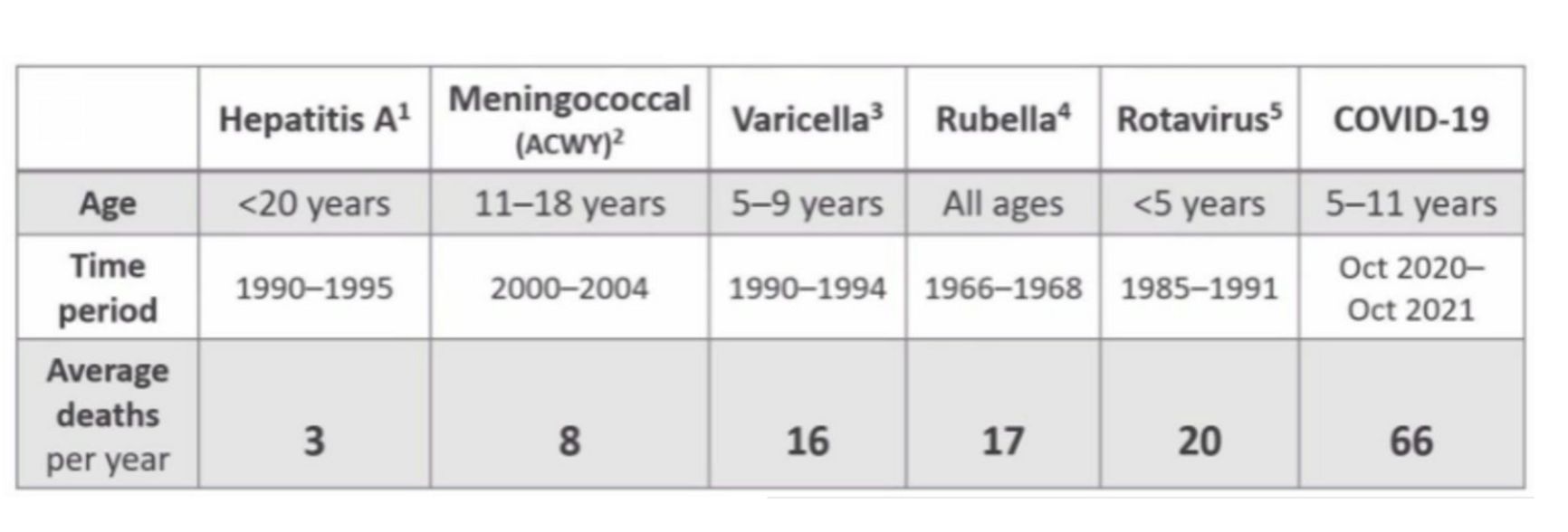
Figure 3. Annual mortality from infectious diseases BEFORE introduction of appropriate vaccines
Of course, it not only the mortality rate that's important: preventing the development of complications and breaking the chain of infection is equally important. In addition, the more people, including children, are vaccinated, the less likely new, more infectious variants of the coronavirus are to emerge.
Figure 4. Modelling the course of the US pandemic as a function of childhood vaccination and the emergence of a new variant of coronavirus. Clearly, childhood vaccination has a particularly pronounced effect when a new variant appears
Nature
The vaccine is recommended for all children, whether or not they have had coronavirus before. Antibody testing before vaccination is not recommended. The only contraindication is anaphylactic reaction to the first dose of the vaccine.
This decision does not mean that other vaccines will automatically be registered in the US or that the Pfizer/BioNTech vaccine will easily overcome regulatory barriers in the European Union and other territories. Each vaccine should be considered separately and carefully, taking into account every available piece of evidence and the local epidemiological situation. So far, WHO recommends vaccinating only children at risk, citing a milder course of covid in children. In addition to the US, vaccination of children under 12 is already underway in Chile, China, the UAE using Chinese vaccines, and in Cuba using the local vaccine.
What about Russia?
Unfortunately, the Russian practice of studying and approving medicines is nothing like the US or European practices, primarily in terms of transparency and expert review of the results. For example, we still have not seen either the Sputnik V study protocol, the Ministry of Health experts report (in violation of Federal Law No. 61), or the clinical trial report.
We still haven't seen either the Sputnik V study protocol or the Ministry of Health's experts report
Nevertheless, the development of a vaccine for children is underway. A reduced-dose version of Sputnik for teens is likely to be registered in November. It will be based on a study involving 99 children aged 12-17, of whom 92 received two doses of the vaccine. No information has been published other than that the vaccine is «capable of inducing good postvaccination cellular immunity» and is safe. For comparison, in the USA, the Pfizer/BioNTech vaccine was registered after a study on 2260 volunteers, of whom 1131 received the vaccine (effectiveness was 100% in preventing the infection). As always, the FDA website offers a wealth of information about the vaccine and its registration process.
However, a third phase is planned for Sputnik involving 3,000 volunteers aged 12-17. Then, studies are likely to be conducted on younger children. There have also been plans to develop a nasal spray vaccine (research has begun on adults) - this will be a highly sought-after product if it proves effective, but the risk of failure is extremely high.
There are also plans to study the EpiVacCorona vaccine on children; it has not been proven effective in adults yet but has been registered by the Ministry of Health.
Vector vaccines, which include Sputnik, have their own safety problems, albeit quite rare - instead of myocarditis, vaccine-induced thrombosis with thrombocytopenia is a concern. Unfortunately, AstraZeneca has stopped pediatric studies of its vaccine, and Johnson & Johnson/Janssen (the closest equivalent of Sputnik) has delayed studies. The experience of using Sputnik on adults in Argentina shows that the incidence of thrombosis after vaccination is no higher than with other vaccines and is probably no higher than the normal incidence of this rare disease in the population. Surprisingly, we have no such data for Russia.
In conclusion, let's hope that more detailed data on pediatric trials of Russian vaccines will be published after all, which can then be evaluated by independent experts. It is also very important that the Pfizer/BioNTech vaccine and other WHO-registered vaccines be allowed into Russia sooner rather than later.
Читать статью на русском